Sharon Strickland
Dr. Sheri Strickland is a Professor of Chemistry who joined the Converse faculty in 2006 and has taught more than 30 different lecture and laboratory courses, including Organic Chemistry I, Organic Chemistry II, Advanced Organic Chemistry, Quantitative Analysis, and the Spectroscopic Identification of Organic Compounds.
Dr. Strickland was awarded the Service to Students Above and Beyond Award (2013), the Converse College Creative and Scholarly Achievement Award (2016), and the Alpha Lambda Delta Favorite Professor Award (2019).
Curriculum Vitae
Read more about Dr. Strickland’s activities and accomplishments:
Scholarly & Research Activity
In the past five years, Dr. Strickland was the co-Principal Investigator on four external grants from the NSF (National Science Foundation), EPSCoR (Established Program to Stimulate Competitive Research, which is a federal-state partnership involving the NSF), and SC-INBRE (South Carolina IDeA Network of Biomedical Research Excellence, which is a federal-state partnership involving the NIH), totaling $250,184. These grants funded the research of eight students, resulting in three peer-reviewed publications in national journals, one peer-reviewed publication in a state journal, and 12 national and regional conference presentations. Two of these four articles and ten of these twelve presentations involve Converse students as co-authors.
Her research focuses on the synthesis and analysis of molecular balances, which are molecules that can exist in two different shapes (or conformations); one conformation can be transformed into the other by rotating around a carbon-nitrogen (C—N) single bond. By measuring the percentage of molecules that choose the “folded” conformation vs. the “unfolded” conformation, we can calculate the amount of stability gained by the intermolecular attraction between the “rotor” and the “shelf” of the molecular balance.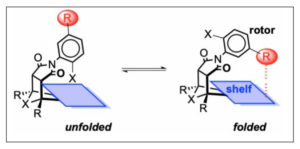
Here’s why that’s important: In the same way that a single water molecule (H2O) is a tiny amount of matter, a single intermolecular attraction is a tiny amount of energy. If a single intermolecular attraction is such a tiny thing, then what difference does it make if we can measure its strength? Consider this: if you collect a trillion trillion water molecules, you can put them into a 1-Liter water bottle and consume about one-fourth of the water that you need daily to stay alive. Similarly, enough of these intermolecular attractions collectively hold proteins in specific shapes, giving them the ability to react in ways that keep us alive. If we can isolate and measure these intermolecular attractions, we will have a better understanding of how to design medications that are more effective and have a longer shelf life.
Selected Publications & Presentations
Dr. Strickland is the co-author of the 2019 textbook Foundations of Organic Chemistry: A Workbook Approach, Volumes I and II and is also the author of two Active Learning activities that resulted from her work as a fellow in the National Science Foundation workshop “Active Learning in Analytical Chemistry” and were published in the Analytical Sciences Digital Library (developed by the National Science Foundation) in 2019. She was also a fellow in the Active Learning in Organic Chemistry workshop (Summer 2021) and has been integrating those concepts and skills into her Organic Chemistry I and Organic Chemistry II courses.